Stucco is generally said to have the property of absorbing formaldehyde and VOCs (volatile organic compounds such as toluene).
Many of you may know this because it is often written in blogs and other media.
However, unless you are quite a maniac about the mechanism, there may be many people who do not know about it.
So this time, for:
- Salespeople at architectural design offices who think "I kind of have an image of adsorption, but I don't really understand it"
- Fathers planning their own homes who are wondering "Why does stucco have an adsorption effect?"
- Interior planners at house makers who think "I want to know the principle of adsorption a little to introduce stucco to customers" I will explain the adsorption mechanism of stucco focusing on images, so please stay with me until the end.
—What is Adsorption?
First, I will explain what adsorption is in the first place.
Wikipedia describes it as follows:
"Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface."
It sounds a bit difficult.
Taking a stucco wall (object) and formaldehyde in the air as an example, it indicates a state where the concentration of formaldehyde increases on the surface (interface) of the stucco wall as shown in the figure below.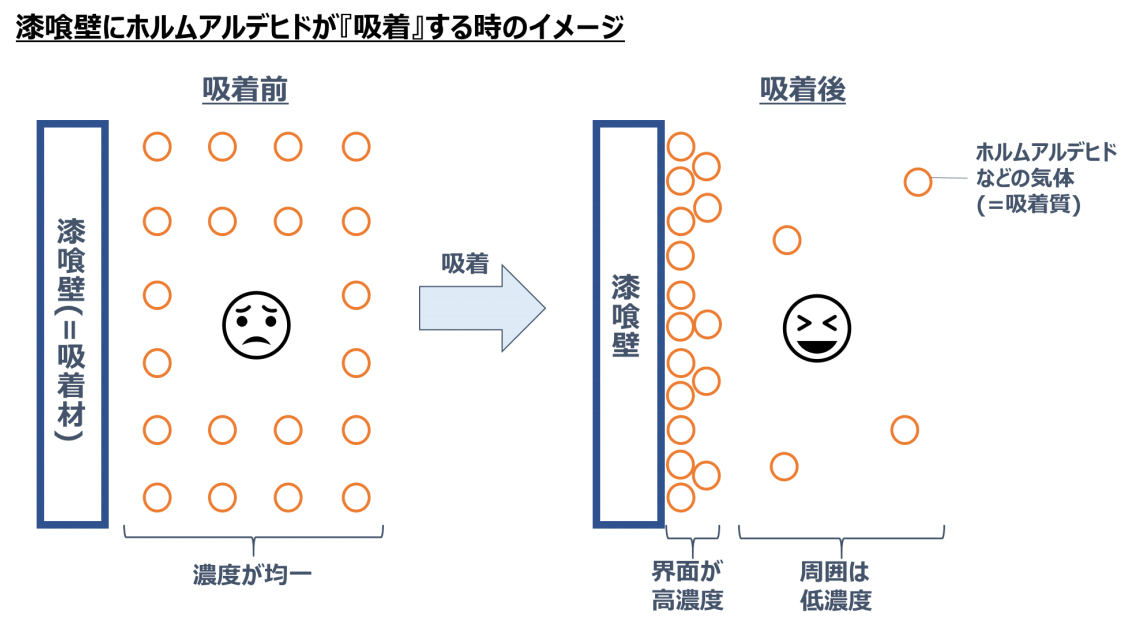
Next, I will explain how adsorption occurs.
—Types of Adsorption: Physical Adsorption and Chemical Adsorption
Adsorption is broadly divided into physical adsorption and chemical adsorption.
I will explain them in order.
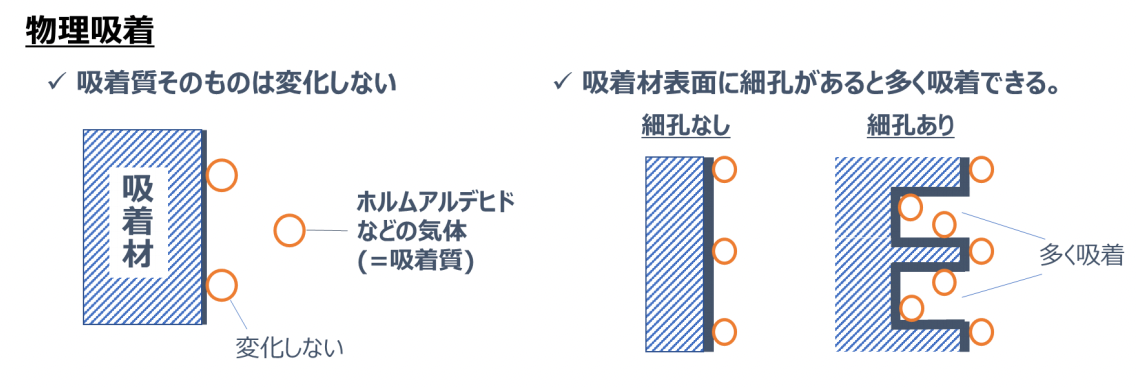
Physical adsorption refers to a phenomenon where an adsorbate sticks to the surface of an adsorbent due to a weak force acting between the adsorbate such as formaldehyde and the adsorbent such as a stucco wall.
Physical adsorption has the following characteristics:
- The adsorbate itself does not change
- If there are very small grooves (pores) on the surface of the adsorbent, many substances can be physically adsorbed
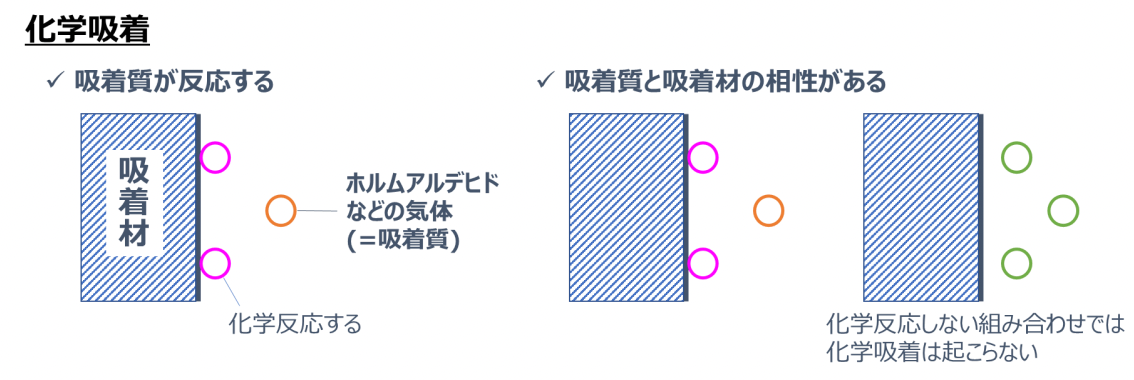
On the other hand, chemical adsorption refers to a phenomenon where an adsorbate such as formaldehyde sticks strongly to the adsorbent surface accompanied by a chemical reaction.
Chemical adsorption has the following characteristics: - The adsorbate reacts and changes into something different
- There is compatibility between the adsorbate and the adsorbent, and chemical adsorption occurs only with adsorbates that chemically react with the adsorbent
So far, I have explained adsorption.
Next, I will explain the main topic of this time, "Why stucco has adsorption properties", the adsorption mechanism of stucco.
—Adsorption Mechanism of Stucco
I will explain the adsorption mechanism of stucco.
The points are "There are many grooves on the surface of stucco" and "It has strong alkaline (minus) properties".
■Stucco bas many grooves on the surface
In the process of drying after application, many grooves are formed on the surface of stucco.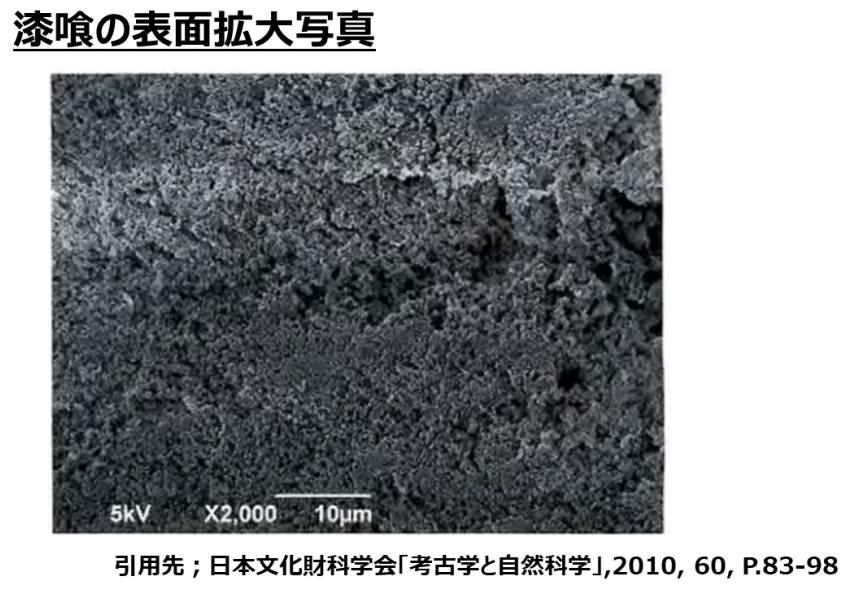
As mentioned earlier, if there are many fine grooves, more substances can be physically adsorbed.
Stucco has the effect of reducing VOCs (volatile organic compounds such as toluene), and the principle is considered to be that VOCs are physically adsorbed in these fine grooves.
■Stucco contains strong alkaline (minus) properties
The main component of stucco is slaked lime (calcium hydroxide).
Slaked lime has very strong alkaline (minus) properties.
Since substances with minus properties and substances with plus properties cause a chemical reaction, substances with plus properties such as acids are chemically adsorbed by stucco.
Stucco has the effect of reducing hydrogen sulfide, which is a component of the smell of rotten eggs, and formaldehyde, and these are chemically adsorbed with slaked lime.
Hydrogen sulfide has hydrogen with acidic (plus) properties, so it reacts with the strong alkali (minus) of slaked lime and becomes an odorless compound.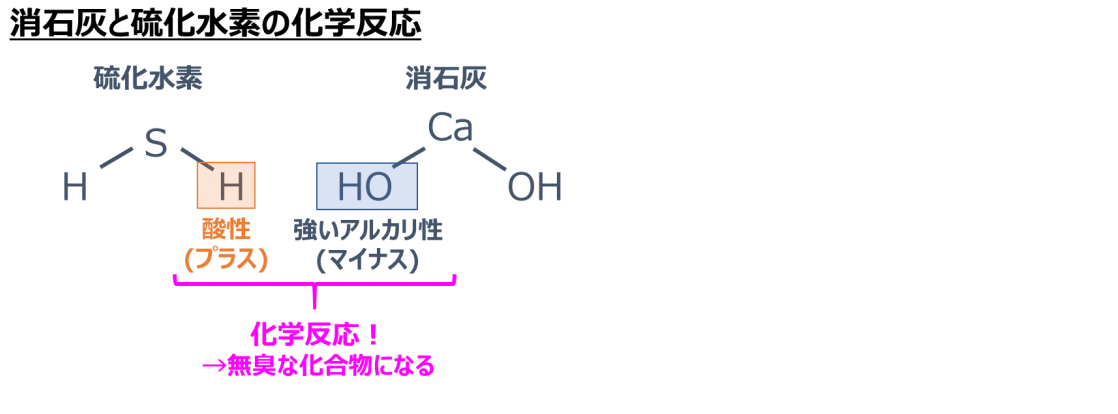
Formaldehyde is a bit complex.
First, a chemical reaction occurs by the action of calcium in slaked lime, resulting in "two formaldehyde post-reaction compounds" described on the right (this process is omitted in the figure below).
That compound has "a little plus hydrogen", and that hydrogen reacts with the strong alkali of slaked lime.
Furthermore, formaldehyde reacts, and finally, harmless sugar is produced.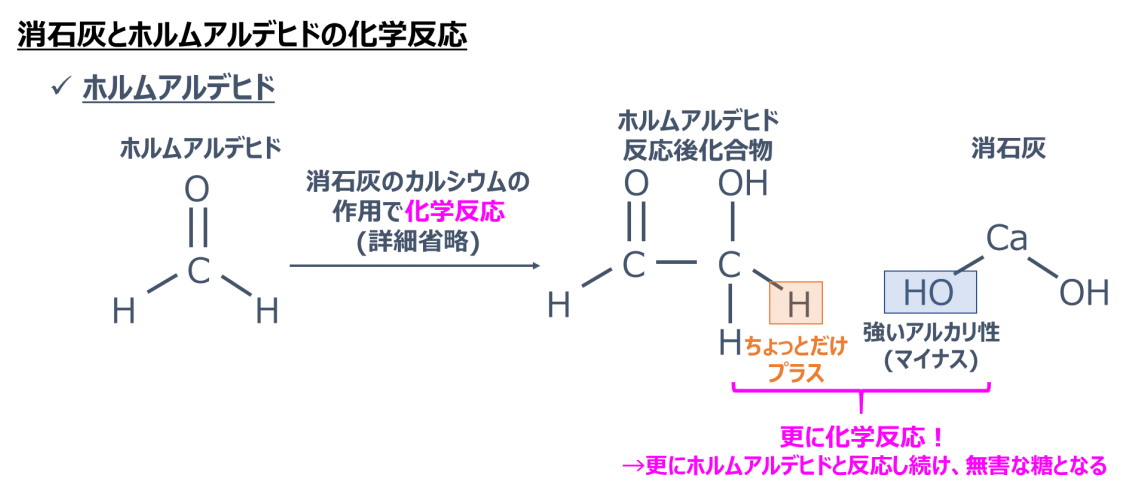
—Summary
This time, I have explained the types of adsorption and why stucco has adsorption power.
■Adsorption includes physical adsorption that does not change the properties of the adsorbed substance, and chemical adsorption that changes the properties.
■Physical adsorption adsorbs well if there are small holes (pores) on the surface.
■Chemical adsorption adsorbs if there is a property to react with the target substance.
■Stucco has the property of physical adsorption because there are many fine holes on the surface, and chemically adsorbs with things with plus properties using its strong minus properties.
I hope you have been able to imagine even a little what is happening in the invisible world of adsorption.



